Abstract

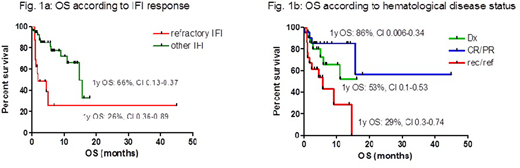
Introduction. The outcome of invasive fungal infections (IFI), particularly aspergillosis in acute leukemia (AL) patients (pts), has improved over the last years, but IFI still remain a major clinical issue in hematological pts, both for its severity and for the toxicity and potential drug interactions of antifungal treatments. Isavuconazole (ISV) is a new antifungal agent, with a modest drug-drug interaction profile, reduced drug-related adverse events and efficacy similar to voriconazole as demonstrated in a non-inferiority trial of IFI treatment (Maertens, Lancet 2016). To confirm the efficacy and safety of ISV in a clinical care setting, we planned a multicenter retrospective epidemiological study on behalf of SEIFEM (Sorveglianza Epidemiologica delle Infezioni nelle Emopatie) Group, collecting all cases of IFI treated with ISV in hematological pts. Patients and Methods. Since July 2016, we collected all cases of IFI occurring in adult and pediatric hematological pts who were treated with ISV in 13 Centers. IFI was diagnosed according to EORTC/MSG criteria and categorized as possible (poss) or probable/proven (p/p). The effects on outcome of age, gender, type and status of hematological disease at IFI (diagnosis [Dx], complete and partial remission [CR/PR], relapse/refractory [Rel/Refr]), type of IFI, allogeneic stem cell transplantation (alloSCT), neutropenia and timing of ISV treatment were evaluated. Results. IFI was diagnosed in 69 pts (M/F ratio: 47/22, median age 54.5y, range 5-80), affected by AL in 49 (71%) (myeloid 35, 51%; lymphoblastic 14, 28%), lymphoma in 15 (22%), myeloma in 2 (3%), severe aplastic anemia in 2 (3%) and myelodysplastic syndromes in 1 (1%) pts, respectively. AlloSCT pts were 20 (29%). IFI were categorized as p/p in 36 (52%) and as poss in 33 (48%) cases. Aspergillus spp was responsible for 31/36 (86%) p/p IFI; in the remaining 5 cases, 1 Rhizomucor pusillus was isolated and in 4, despite histology proven for IFI, no specific agent could be identified. Lung was the more frequent site of IFI (58/69, 84%), followed by paranasal sinuses (5, 7%), liver (3, 5%), brain (2, 3%), and bone (1, 1%). ISV was employed as first-line therapy in 19 (28%) and as subsequent line of treatment in 50 pts (72%; 41 as second and 9 as subsequent, respectively), following voriconazole in 15 cases, L-AmB in 22, caspofungin in 1 and combination therapy in 12. The median duration of previous treatments was 17.5 days (range 5-1110). Reasons for ISV use were failure of previous treatments in 16 (32%) and intolerance in 17 (34%) of 50 cases, respectively. In 16 cases (32%) ISV was chosen because of the need to switch antifungal treatment to an oral agent for outpatients and in 3 (4%) for a favorable drug-drug interaction profile. Median duration of ISV treatment was 60 days (3-210). After a median follow-up of 4.2 mo, 24/69 (35%) pts died; IFI-attributable mortality was 10/69 (15%). The estimated 1-year overall survival (OS) from IFI event of the entire cohort was 56% (CI 0.22-0.45); it was similar when considering poss vs p/p IFI (62% vs 51%, p=0.39), AL vs no AL (51% vs 69%, p=0.395) and first vs subsequent line use of ISV (66% vs 59%, p=0.6). OS was significantly lower for pts with failure to previous treatments (1y OS: refractory IFI 26% vs not refractory IFI 66%, p=0.001) (Fig. 1a) and for pts with rec/ref hematological disease (1y OS: rec/ref 29% vs CR/PR 86%, p=0.0027, or vs Dx 53%, p=0.06; no differences between Dx and CR/PR, p=0.27) (Fig. 1b). Clinical and radiological overall response rate (ORR) was 44 of 64 evaluable pts (69%). In multivariate analysis, only underlying disease status was a predictive factor for response to ISV (rec/ref HR 0.202, CI 0.062-0.658). Adverse events (AE) were reported in 10/69 pts (15%) (hepatic in 5, cutaneous in 3, gastroenteric in 3 and hypokaliemia in 1); grade 3-4 AE were reported in 5 cases and led to permanent ISV discontinuation. Conclusions. ISV is widely used in hematological pts with IFI also in diseases other than acute myeloid leukemia and it is overall well tolerated. ORR to ISV is at least comparable with other antifungal agents. A rec/ref underlying hematological disease impacts both on OS and response to ISV, while having an IFI refractory to other antifungal agents including azoles does not seem to compromise the response to ISV, although this promising result should be confirmed in prospective studies and larger groups of patients.
Busca:Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Merk: Honoraria, Speakers Bureau; Pfizer Pharmaceuticals: Honoraria, Speakers Bureau; Jazz Pharmaceuticals: Honoraria; Novartis: Speakers Bureau. Fanci:Gilead: Honoraria; Pfizer Pharmaceuticals: Honoraria; Merck: Consultancy, Honoraria, Speakers Bureau. Candoni:Pfizer: Honoraria, Speakers Bureau; Merck SD: Honoraria, Speakers Bureau; Celgene: Honoraria, Speakers Bureau; Janssen: Honoraria, Speakers Bureau; Gilead: Honoraria, Speakers Bureau. Fracchiolla:Amgen: Membership on an entity's Board of Directors or advisory committees; Merck: Honoraria, Speakers Bureau; Pfizer Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Tumbarello:MSD: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Angelini: Consultancy, Membership on an entity's Board of Directors or advisory committees; Roche: Consultancy, Membership on an entity's Board of Directors or advisory committees; Nordic Pharma: Membership on an entity's Board of Directors or advisory committees; Astellas: Speakers Bureau; Pfizer: Speakers Bureau. Aversa:Merck: Honoraria; Basilea: Honoraria, Membership on an entity's Board of Directors or advisory committees; Astellas: Honoraria; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees. Rossi:Amgen: Membership on an entity's Board of Directors or advisory committees; Jazz: Membership on an entity's Board of Directors or advisory committees; Mundipharma: Honoraria; Novartis: Honoraria; Celgene: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees; BMS: Honoraria; Sandoz: Honoraria; Pfizer: Membership on an entity's Board of Directors or advisory committees; Teva: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees. Pagano:Basilea: Speakers Bureau; Merck: Speakers Bureau; Janssen: Speakers Bureau; Pfizer: Speakers Bureau; Gilead: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract